LIST OF TABLES
TABLE 01. GLOBAL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 02. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR RILUZOLE, BY REGION, 2022-2032 ($MILLION)
TABLE 03. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR EDARAVONE, BY REGION, 2022-2032 ($MILLION)
TABLE 04. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
TABLE 05. GLOBAL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 06. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR SPORADIC ALS, BY REGION, 2022-2032 ($MILLION)
TABLE 07. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR FAMILIAL ALS, BY REGION, 2022-2032 ($MILLION)
TABLE 08. GLOBAL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 09. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR HOSPITAL PHARMACIES, BY REGION, 2022-2032 ($MILLION)
TABLE 10. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR ONLINE PROVIDERS, BY REGION, 2022-2032 ($MILLION)
TABLE 11. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR DRUG STORES AND RETAIL PHARMACIES, BY REGION, 2022-2032 ($MILLION)
TABLE 12. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY REGION, 2022-2032 ($MILLION)
TABLE 13. NORTH AMERICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 14. NORTH AMERICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 15. NORTH AMERICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 16. NORTH AMERICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY COUNTRY, 2022-2032 ($MILLION)
TABLE 17. U.S. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 18. U.S. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 19. U.S. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 20. CANADA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 21. CANADA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 22. CANADA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 23. MEXICO AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 24. MEXICO AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 25. MEXICO AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 26. EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 27. EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 28. EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 29. EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY COUNTRY, 2022-2032 ($MILLION)
TABLE 30. GERMANY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 31. GERMANY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 32. GERMANY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 33. FRANCE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 34. FRANCE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 35. FRANCE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 36. UK AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 37. UK AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 38. UK AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 39. ITALY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 40. ITALY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 41. ITALY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 42. SPAIN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 43. SPAIN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 44. SPAIN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 45. REST OF EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 46. REST OF EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 47. REST OF EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 48. ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 49. ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 50. ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 51. ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY COUNTRY, 2022-2032 ($MILLION)
TABLE 52. JAPAN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 53. JAPAN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 54. JAPAN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 55. CHINA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 56. CHINA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 57. CHINA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 58. AUSTRALIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 59. AUSTRALIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 60. AUSTRALIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 61. INDIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 62. INDIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 63. INDIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 64. SOUTH KOREA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 65. SOUTH KOREA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 66. SOUTH KOREA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 67. REST OF ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 68. REST OF ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 69. REST OF ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 70. LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 71. LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 72. LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 73. LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY COUNTRY, 2022-2032 ($MILLION)
TABLE 74. BRAZIL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 75. BRAZIL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 76. BRAZIL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 77. SAUDI ARABIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 78. SAUDI ARABIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 79. SAUDI ARABIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 80. SOUTH AFRICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 81. SOUTH AFRICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 82. SOUTH AFRICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 83. REST OF LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022-2032 ($MILLION)
TABLE 84. REST OF LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022-2032 ($MILLION)
TABLE 85. REST OF LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022-2032 ($MILLION)
TABLE 86. AMYLYX PHARMACEUTICALS, INC.: KEY EXECUTIVES
TABLE 87. AMYLYX PHARMACEUTICALS, INC.: COMPANY SNAPSHOT
TABLE 88. AMYLYX PHARMACEUTICALS, INC.: PRODUCT SEGMENTS
TABLE 89. AMYLYX PHARMACEUTICALS, INC.: PRODUCT PORTFOLIO
TABLE 90. AMYLYX PHARMACEUTICALS, INC.: KEY STRATERGIES
TABLE 91. AQUESTIVE THERAPEUTICS, INC.: KEY EXECUTIVES
TABLE 92. AQUESTIVE THERAPEUTICS, INC.: COMPANY SNAPSHOT
TABLE 93. AQUESTIVE THERAPEUTICS, INC.: PRODUCT SEGMENTS
TABLE 94. AQUESTIVE THERAPEUTICS, INC.: PRODUCT PORTFOLIO
TABLE 95. COVIS PHARMA GMBH: KEY EXECUTIVES
TABLE 96. COVIS PHARMA GMBH: COMPANY SNAPSHOT
TABLE 97. COVIS PHARMA GMBH: PRODUCT SEGMENTS
TABLE 98. COVIS PHARMA GMBH: PRODUCT PORTFOLIO
TABLE 99. GLENMARK PHARMACEUTICALS LIMITED: KEY EXECUTIVES
TABLE 100. GLENMARK PHARMACEUTICALS LIMITED: COMPANY SNAPSHOT
TABLE 101. GLENMARK PHARMACEUTICALS LIMITED: PRODUCT SEGMENTS
TABLE 102. GLENMARK PHARMACEUTICALS LIMITED: PRODUCT PORTFOLIO
TABLE 103. ITALFARMACO S.P.A.: KEY EXECUTIVES
TABLE 104. ITALFARMACO S.P.A.: COMPANY SNAPSHOT
TABLE 105. ITALFARMACO S.P.A.: PRODUCT SEGMENTS
TABLE 106. ITALFARMACO S.P.A.: PRODUCT PORTFOLIO
TABLE 107. MITSUBISHI CHEMICAL GROUP CORPORATION: KEY EXECUTIVES
TABLE 108. MITSUBISHI CHEMICAL GROUP CORPORATION: COMPANY SNAPSHOT
TABLE 109. MITSUBISHI CHEMICAL GROUP CORPORATION: PRODUCT SEGMENTS
TABLE 110. MITSUBISHI CHEMICAL GROUP CORPORATION: PRODUCT PORTFOLIO
TABLE 111. MITSUBISHI CHEMICAL GROUP CORPORATION: KEY STRATERGIES
TABLE 112. SUN PHARMACEUTICAL INDUSTRIES LTD.: KEY EXECUTIVES
TABLE 113. SUN PHARMACEUTICAL INDUSTRIES LTD.: COMPANY SNAPSHOT
TABLE 114. SUN PHARMACEUTICAL INDUSTRIES LTD.: PRODUCT SEGMENTS
TABLE 115. SUN PHARMACEUTICAL INDUSTRIES LTD.: PRODUCT PORTFOLIO
TABLE 116. VIATRIS INC.: KEY EXECUTIVES
TABLE 117. VIATRIS INC.: COMPANY SNAPSHOT
TABLE 118. VIATRIS INC.: PRODUCT SEGMENTS
TABLE 119. VIATRIS INC.: PRODUCT PORTFOLIO
TABLE 120. ALKEM LABORATORIES LTD: KEY EXECUTIVES
TABLE 121. ALKEM LABORATORIES LTD: COMPANY SNAPSHOT
TABLE 122. ALKEM LABORATORIES LTD: PRODUCT SEGMENTS
TABLE 123. ALKEM LABORATORIES LTD: PRODUCT PORTFOLIO
TABLE 124. OTSUKA PHARMACEUTICAL CO., LTD.: KEY EXECUTIVES
TABLE 125. OTSUKA PHARMACEUTICAL CO., LTD.: COMPANY SNAPSHOT
TABLE 126. OTSUKA PHARMACEUTICAL CO., LTD.: PRODUCT SEGMENTS
TABLE 127. OTSUKA PHARMACEUTICAL CO., LTD.: PRODUCT PORTFOLIO LIST OF FIGURES
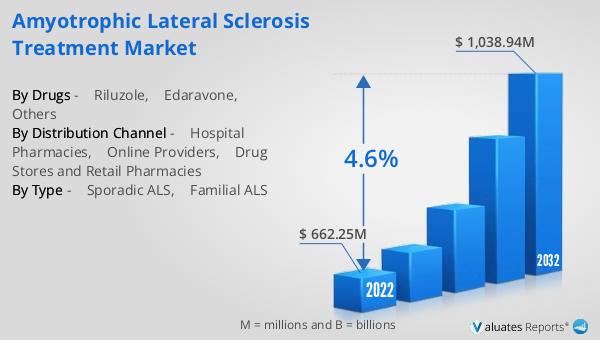
FIGURE 01. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032
FIGURE 02. SEGMENTATION OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032
FIGURE 03. TOP INVESTMENT POCKETS IN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET (2023-2032)
FIGURE 04. MODERATE BARGAINING POWER OF SUPPLIERS
FIGURE 05. MODERATE BARGAINING POWER OF BUYERS
FIGURE 06. MODERATE THREAT OF SUBSTITUTES
FIGURE 07. MODERATE THREAT OF NEW ENTRANTS
FIGURE 08. MODERATE INTENSITY OF RIVALRY
FIGURE 09. DRIVERS, RESTRAINTS AND OPPORTUNITIES: GLOBALAMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET
FIGURE 10. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DRUGS, 2022(%)
FIGURE 11. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR RILUZOLE, BY COUNTRY 2022 AND 2032(%)
FIGURE 12. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR EDARAVONE, BY COUNTRY 2022 AND 2032(%)
FIGURE 13. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
FIGURE 14. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY TYPE, 2022(%)
FIGURE 15. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR SPORADIC ALS, BY COUNTRY 2022 AND 2032(%)
FIGURE 16. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR FAMILIAL ALS, BY COUNTRY 2022 AND 2032(%)
FIGURE 17. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2022(%)
FIGURE 18. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR HOSPITAL PHARMACIES, BY COUNTRY 2022 AND 2032(%)
FIGURE 19. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR ONLINE PROVIDERS, BY COUNTRY 2022 AND 2032(%)
FIGURE 20. COMPARATIVE SHARE ANALYSIS OF AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET FOR DRUG STORES AND RETAIL PHARMACIES, BY COUNTRY 2022 AND 2032(%)
FIGURE 21. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET BY REGION, 2022
FIGURE 22. U.S. AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 23. CANADA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 24. MEXICO AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 25. GERMANY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 26. FRANCE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 27. UK AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 28. ITALY AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 29. SPAIN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 30. REST OF EUROPE AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 31. JAPAN AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 32. CHINA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 33. AUSTRALIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 34. INDIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 35. SOUTH KOREA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 36. REST OF ASIA-PACIFIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 37. BRAZIL AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 38. SAUDI ARABIA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 39. SOUTH AFRICA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 40. REST OF LAMEA AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET, 2022-2032 ($MILLION)
FIGURE 41. TOP WINNING STRATEGIES, BY YEAR
FIGURE 42. TOP WINNING STRATEGIES, BY DEVELOPMENT
FIGURE 43. TOP WINNING STRATEGIES, BY COMPANY
FIGURE 44. PRODUCT MAPPING OF TOP 10 PLAYERS
FIGURE 45. COMPETITIVE DASHBOARD
FIGURE 46. COMPETITIVE HEATMAP: AMYOTROPHIC LATERAL SCLEROSIS TREATMENT MARKET
FIGURE 47. TOP PLAYER POSITIONING, 2022
FIGURE 48. AMYLYX PHARMACEUTICALS, INC.: NET REVENUE, 2020-2022 ($MILLION)
FIGURE 49. AQUESTIVE THERAPEUTICS, INC.: NET REVENUE, 2020-2022 ($MILLION)
FIGURE 50. AQUESTIVE THERAPEUTICS, INC.: REVENUE SHARE BY REGION, 2022 (%)
FIGURE 51. GLENMARK PHARMACEUTICALS LIMITED: NET SALES, 2020-2022 ($MILLION)
FIGURE 52. GLENMARK PHARMACEUTICALS LIMITED: REVENUE SHARE BY REGION, 2022 (%)
FIGURE 53. MITSUBISHI CHEMICAL GROUP CORPORATION: NET SALES, 2020-2022 ($MILLION)
FIGURE 54. MITSUBISHI CHEMICAL GROUP CORPORATION: REVENUE SHARE BY SEGMENT, 2022 (%)
FIGURE 55. SUN PHARMACEUTICAL INDUSTRIES LTD.: NET REVENUE, 2019-2021 ($MILLION)
FIGURE 56. SUN PHARMACEUTICAL INDUSTRIES LTD.: REVENUE SHARE BY REGION, 2021 (%)
FIGURE 57. VIATRIS INC.: NET SALES, 2020-2022 ($MILLION)
FIGURE 58. VIATRIS INC.: REVENUE SHARE BY SEGMENT, 2022 (%)
FIGURE 59. ALKEM LABORATORIES LTD.: NET REVENUE, 2020-2022 ($MILLION)
FIGURE 60. ALKEM LABORATORIES LTD.: REVENUE SHARE BY REGION, 2022 (%