List of Tables
Table 1. Global Lab Automation for In-vitro Diagnostics Market Value by Type, (US$ Million) & (2023 VS 2030)
Table 2. Global Lab Automation for In-vitro Diagnostics Market Value by Application, (US$ Million) & (2023 VS 2030)
Table 3. Global Lab Automation for In-vitro Diagnostics Production Capacity (K Units) by Manufacturers in 2023
Table 4. Global Lab Automation for In-vitro Diagnostics Production by Manufacturers (2019-2024) & (K Units)
Table 5. Global Lab Automation for In-vitro Diagnostics Production Market Share by Manufacturers (2019-2024)
Table 6. Global Lab Automation for In-vitro Diagnostics Production Value by Manufacturers (2019-2024) & (US$ Million)
Table 7. Global Lab Automation for In-vitro Diagnostics Production Value Share by Manufacturers (2019-2024)
Table 8. Global Lab Automation for In-vitro Diagnostics Industry Ranking 2022 VS 2023 VS 2024
Table 9. Company Type (Tier 1, Tier 2 and Tier 3) & (based on the Revenue in Lab Automation for In-vitro Diagnostics as of 2023)
Table 10. Global Market Lab Automation for In-vitro Diagnostics Average Price by Manufacturers (USD/Unit) & (2019-2024)
Table 11. Manufacturers Lab Automation for In-vitro Diagnostics Production Sites and Area Served
Table 12. Manufacturers Lab Automation for In-vitro Diagnostics Product Types
Table 13. Global Lab Automation for In-vitro Diagnostics Manufacturers Market Concentration Ratio (CR5 and HHI)
Table 14. Mergers & Acquisitions, Expansion
Table 15. Global Lab Automation for In-vitro Diagnostics Production Value by Region: 2019 VS 2023 VS 2030 (US$ Million)
Table 16. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) by Region (2019-2024)
Table 17. Global Lab Automation for In-vitro Diagnostics Production Value Market Share by Region (2019-2024)
Table 18. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) Forecast by Region (2025-2030)
Table 19. Global Lab Automation for In-vitro Diagnostics Production Value Market Share Forecast by Region (2025-2030)
Table 20. Global Lab Automation for In-vitro Diagnostics Production Comparison by Region: 2019 VS 2023 VS 2030 (K Units)
Table 21. Global Lab Automation for In-vitro Diagnostics Production (K Units) by Region (2019-2024)
Table 22. Global Lab Automation for In-vitro Diagnostics Production Market Share by Region (2019-2024)
Table 23. Global Lab Automation for In-vitro Diagnostics Production (K Units) Forecast by Region (2025-2030)
Table 24. Global Lab Automation for In-vitro Diagnostics Production Market Share Forecast by Region (2025-2030)
Table 25. Global Lab Automation for In-vitro Diagnostics Market Average Price (USD/Unit) by Region (2019-2024)
Table 26. Global Lab Automation for In-vitro Diagnostics Market Average Price (USD/Unit) by Region (2025-2030)
Table 27. Global Lab Automation for In-vitro Diagnostics Consumption Growth Rate by Region: 2019 VS 2023 VS 2030 (K Units)
Table 28. Global Lab Automation for In-vitro Diagnostics Consumption by Region (2019-2024) & (K Units)
Table 29. Global Lab Automation for In-vitro Diagnostics Consumption Market Share by Region (2019-2024)
Table 30. Global Lab Automation for In-vitro Diagnostics Forecasted Consumption by Region (2025-2030) & (K Units)
Table 31. Global Lab Automation for In-vitro Diagnostics Forecasted Consumption Market Share by Region (2019-2024)
Table 32. North America Lab Automation for In-vitro Diagnostics Consumption Growth Rate by Country: 2019 VS 2023 VS 2030 (K Units)
Table 33. North America Lab Automation for In-vitro Diagnostics Consumption by Country (2019-2024) & (K Units)
Table 34. North America Lab Automation for In-vitro Diagnostics Consumption by Country (2025-2030) & (K Units)
Table 35. Europe Lab Automation for In-vitro Diagnostics Consumption Growth Rate by Country: 2019 VS 2023 VS 2030 (K Units)
Table 36. Europe Lab Automation for In-vitro Diagnostics Consumption by Country (2019-2024) & (K Units)
Table 37. Europe Lab Automation for In-vitro Diagnostics Consumption by Country (2025-2030) & (K Units)
Table 38. Asia Pacific Lab Automation for In-vitro Diagnostics Consumption Growth Rate by Region: 2019 VS 2023 VS 2030 (K Units)
Table 39. Asia Pacific Lab Automation for In-vitro Diagnostics Consumption by Region (2019-2024) & (K Units)
Table 40. Asia Pacific Lab Automation for In-vitro Diagnostics Consumption by Region (2025-2030) & (K Units)
Table 41. Latin America, Middle East & Africa Lab Automation for In-vitro Diagnostics Consumption Growth Rate by Country: 2019 VS 2023 VS 2030 (K Units)
Table 42. Latin America, Middle East & Africa Lab Automation for In-vitro Diagnostics Consumption by Country (2019-2024) & (K Units)
Table 43. Latin America, Middle East & Africa Lab Automation for In-vitro Diagnostics Consumption by Country (2025-2030) & (K Units)
Table 44. Global Lab Automation for In-vitro Diagnostics Production (K Units) by Type (2019-2024)
Table 45. Global Lab Automation for In-vitro Diagnostics Production (K Units) by Type (2025-2030)
Table 46. Global Lab Automation for In-vitro Diagnostics Production Market Share by Type (2019-2024)
Table 47. Global Lab Automation for In-vitro Diagnostics Production Market Share by Type (2025-2030)
Table 48. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) by Type (2019-2024)
Table 49. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) by Type (2025-2030)
Table 50. Global Lab Automation for In-vitro Diagnostics Production Value Share by Type (2019-2024)
Table 51. Global Lab Automation for In-vitro Diagnostics Production Value Share by Type (2025-2030)
Table 52. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Type (2019-2024)
Table 53. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Type (2025-2030)
Table 54. Global Lab Automation for In-vitro Diagnostics Production (K Units) by Application (2019-2024)
Table 55. Global Lab Automation for In-vitro Diagnostics Production (K Units) by Application (2025-2030)
Table 56. Global Lab Automation for In-vitro Diagnostics Production Market Share by Application (2019-2024)
Table 57. Global Lab Automation for In-vitro Diagnostics Production Market Share by Application (2025-2030)
Table 58. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) by Application (2019-2024)
Table 59. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) by Application (2025-2030)
Table 60. Global Lab Automation for In-vitro Diagnostics Production Value Share by Application (2019-2024)
Table 61. Global Lab Automation for In-vitro Diagnostics Production Value Share by Application (2025-2030)
Table 62. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Application (2019-2024)
Table 63. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Application (2025-2030)
Table 64. Cognex Corporation Lab Automation for In-vitro Diagnostics Corporation Information
Table 65. Cognex Corporation Specification and Application
Table 66. Cognex Corporation Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 67. Cognex Corporation Main Business and Markets Served
Table 68. Cognex Corporation Recent Developments/Updates
Table 69. F. Hoffmann-La Roche Ltd Lab Automation for In-vitro Diagnostics Corporation Information
Table 70. F. Hoffmann-La Roche Ltd Specification and Application
Table 71. F. Hoffmann-La Roche Ltd Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 72. F. Hoffmann-La Roche Ltd Main Business and Markets Served
Table 73. F. Hoffmann-La Roche Ltd Recent Developments/Updates
Table 74. Thermo Fisher Scientific Inc Lab Automation for In-vitro Diagnostics Corporation Information
Table 75. Thermo Fisher Scientific Inc Specification and Application
Table 76. Thermo Fisher Scientific Inc Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 77. Thermo Fisher Scientific Inc Main Business and Markets Served
Table 78. Thermo Fisher Scientific Inc Recent Developments/Updates
Table 79. Danaher Corporation Lab Automation for In-vitro Diagnostics Corporation Information
Table 80. Danaher Corporation Specification and Application
Table 81. Danaher Corporation Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 82. Danaher Corporation Main Business and Markets Served
Table 83. Danaher Corporation Recent Developments/Updates
Table 84. Agilent Technologies, Inc Lab Automation for In-vitro Diagnostics Corporation Information
Table 85. Agilent Technologies, Inc Specification and Application
Table 86. Agilent Technologies, Inc Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 87. Agilent Technologies, Inc Main Business and Markets Served
Table 88. Agilent Technologies, Inc Recent Developments/Updates
Table 89. Abbott Lab Automation for In-vitro Diagnostics Corporation Information
Table 90. Abbott Specification and Application
Table 91. Abbott Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 92. Abbott Main Business and Markets Served
Table 93. Abbott Recent Developments/Updates
Table 94. PerkinElmer, Inc Lab Automation for In-vitro Diagnostics Corporation Information
Table 95. PerkinElmer, Inc Specification and Application
Table 96. PerkinElmer, Inc Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 97. PerkinElmer, Inc Main Business and Markets Served
Table 98. PerkinElmer, Inc Recent Developments/Updates
Table 99. Tecan Group Ltd Lab Automation for In-vitro Diagnostics Corporation Information
Table 100. Tecan Group Ltd Specification and Application
Table 101. Tecan Group Ltd Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 102. Tecan Group Ltd Main Business and Markets Served
Table 103. Tecan Group Ltd Recent Developments/Updates
Table 104. BD Lab Automation for In-vitro Diagnostics Corporation Information
Table 105. BD Specification and Application
Table 106. BD Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 107. BD Main Business and Markets Served
Table 108. BD Recent Developments/Updates
Table 109. Siemens Lab Automation for In-vitro Diagnostics Corporation Information
Table 110. Siemens Specification and Application
Table 111. Siemens Lab Automation for In-vitro Diagnostics Production (K Units), Value (US$ Million), Price (USD/Unit) and Gross Margin (2019-2024)
Table 112. Siemens Main Business and Markets Served
Table 113. Siemens Recent Developments/Updates
Table 114. Key Raw Materials Lists
Table 115. Raw Materials Key Suppliers Lists
Table 116. Lab Automation for In-vitro Diagnostics Distributors List
Table 117. Lab Automation for In-vitro Diagnostics Customers List
Table 118. Lab Automation for In-vitro Diagnostics Market Trends
Table 119. Lab Automation for In-vitro Diagnostics Market Drivers
Table 120. Lab Automation for In-vitro Diagnostics Market Challenges
Table 121. Lab Automation for In-vitro Diagnostics Market Restraints
Table 122. Research Programs/Design for This Report
Table 123. Key Data Information from Secondary Sources
Table 124. Key Data Information from Primary Sources
List of Figures
Figure 1. Product Picture of Lab Automation for In-vitro Diagnostics
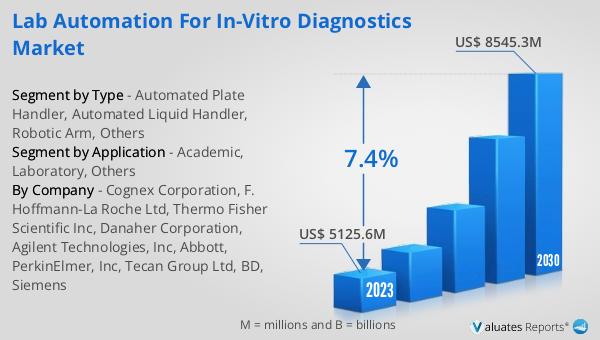
Figure 2. Global Lab Automation for In-vitro Diagnostics Market Value by Type, (US$ Million) & (2023 VS 2030)
Figure 3. Global Lab Automation for In-vitro Diagnostics Market Share by Type: 2023 VS 2030
Figure 4. Automated Plate Handler Product Picture
Figure 5. Automated Liquid Handler Product Picture
Figure 6. Robotic Arm Product Picture
Figure 7. Others Product Picture
Figure 8. Global Lab Automation for In-vitro Diagnostics Market Value by Application, (US$ Million) & (2023 VS 2030)
Figure 9. Global Lab Automation for In-vitro Diagnostics Market Share by Application: 2023 VS 2030
Figure 10. Academic
Figure 11. Laboratory
Figure 12. Others
Figure 13. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million), 2019 VS 2023 VS 2030
Figure 14. Global Lab Automation for In-vitro Diagnostics Production Value (US$ Million) & (2019-2030)
Figure 15. Global Lab Automation for In-vitro Diagnostics Production (K Units) & (2019-2030)
Figure 16. Global Lab Automation for In-vitro Diagnostics Average Price (USD/Unit) & (2019-2030)
Figure 17. Lab Automation for In-vitro Diagnostics Report Years Considered
Figure 18. Lab Automation for In-vitro Diagnostics Production Share by Manufacturers in 2023
Figure 19. Lab Automation for In-vitro Diagnostics Market Share by Company Type (Tier 1, Tier 2, and Tier 3): 2019 VS 2023
Figure 20. The Global 5 and 10 Largest Players: Market Share by Lab Automation for In-vitro Diagnostics Revenue in 2023
Figure 21. Global Lab Automation for In-vitro Diagnostics Production Value by Region: 2019 VS 2023 VS 2030 (US$ Million)
Figure 22. Global Lab Automation for In-vitro Diagnostics Production Value Market Share by Region: 2019 VS 2023 VS 2030
Figure 23. Global Lab Automation for In-vitro Diagnostics Production Comparison by Region: 2019 VS 2023 VS 2030 (K Units)
Figure 24. Global Lab Automation for In-vitro Diagnostics Production Market Share by Region: 2019 VS 2023 VS 2030
Figure 25. North America Lab Automation for In-vitro Diagnostics Production Value (US$ Million) Growth Rate (2019-2030)
Figure 26. Europe Lab Automation for In-vitro Diagnostics Production Value (US$ Million) Growth Rate (2019-2030)
Figure 27. Asia-Pacific Lab Automation for In-vitro Diagnostics Production Value (US$ Million) Growth Rate (2019-2030)
Figure 28. Global Lab Automation for In-vitro Diagnostics Consumption by Region: 2019 VS 2023 VS 2030 (K Units)
Figure 29. Global Lab Automation for In-vitro Diagnostics Consumption Market Share by Region: 2019 VS 2023 VS 2030
Figure 30. North America Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 31. North America Lab Automation for In-vitro Diagnostics Consumption Market Share by Country (2019-2030)
Figure 32. Canada Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 33. U.S. Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 34. Europe Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 35. Europe Lab Automation for In-vitro Diagnostics Consumption Market Share by Country (2019-2030)
Figure 36. Germany Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 37. France Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 38. U.K. Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 39. Italy Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 40. Russia Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 41. Asia Pacific Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 42. Asia Pacific Lab Automation for In-vitro Diagnostics Consumption Market Share by Regions (2019-2030)
Figure 43. China Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 44. Japan Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 45. South Korea Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 46. China Taiwan Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 47. Southeast Asia Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 48. India Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 49. Latin America, Middle East & Africa Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 50. Latin America, Middle East & Africa Lab Automation for In-vitro Diagnostics Consumption Market Share by Country (2019-2030)
Figure 51. Mexico Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 52. Brazil Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 53. Turkey Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 54. GCC Countries Lab Automation for In-vitro Diagnostics Consumption and Growth Rate (2019-2024) & (K Units)
Figure 55. Global Production Market Share of Lab Automation for In-vitro Diagnostics by Type (2019-2030)
Figure 56. Global Production Value Market Share of Lab Automation for In-vitro Diagnostics by Type (2019-2030)
Figure 57. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Type (2019-2030)
Figure 58. Global Production Market Share of Lab Automation for In-vitro Diagnostics by Application (2019-2030)
Figure 59. Global Production Value Market Share of Lab Automation for In-vitro Diagnostics by Application (2019-2030)
Figure 60. Global Lab Automation for In-vitro Diagnostics Price (USD/Unit) by Application (2019-2030)
Figure 61. Lab Automation for In-vitro Diagnostics Value Chain
Figure 62. Lab Automation for In-vitro Diagnostics Production Process
Figure 63. Channels of Distribution (Direct Vs Distribution)
Figure 64. Distributors Profiles
Figure 65. Bottom-up and Top-down Approaches for This Report
Figure 66. Data Triangulation