List of Tables
Table 1. Global Medical Device Bioburden Testing Market Size Growth Rate by Type (US$ Million): 2020 VS 2024 VS 2031
Table 2. Key Players of Membrane Filtration Method
Table 3. Key Players of Most-Probable-Number Method
Table 4. Key Players of Pour-Plate Method
Table 5. Key Players of Surface-Spread Method
Table 6. Global Medical Device Bioburden Testing Market Size Growth by Application (US$ Million): 2020 VS 2024 VS 2031
Table 7. Global Medical Device Bioburden Testing Market Size by Region (US$ Million): 2020 VS 2024 VS 2031
Table 8. Global Medical Device Bioburden Testing Market Size by Region (2020-2025) & (US$ Million)
Table 9. Global Medical Device Bioburden Testing Market Share by Region (2020-2025)
Table 10. Global Medical Device Bioburden Testing Forecasted Market Size by Region (2026-2031) & (US$ Million)
Table 11. Global Medical Device Bioburden Testing Market Share by Region (2026-2031)
Table 12. Medical Device Bioburden Testing Market Trends
Table 13. Medical Device Bioburden Testing Market Drivers
Table 14. Medical Device Bioburden Testing Market Challenges
Table 15. Medical Device Bioburden Testing Market Restraints
Table 16. Global Medical Device Bioburden Testing Revenue by Players (2020-2025) & (US$ Million)
Table 17. Global Medical Device Bioburden Testing Market Share by Players (2020-2025)
Table 18. Global Top Medical Device Bioburden Testing Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Medical Device Bioburden Testing as of 2024)
Table 19. Ranking of Global Top Medical Device Bioburden Testing Companies by Revenue (US$ Million) in 2024
Table 20. Global 5 Largest Players Market Share by Medical Device Bioburden Testing Revenue (CR5 and HHI) & (2020-2025)
Table 21. Global Key Players of Medical Device Bioburden Testing, Headquarters and Area Served
Table 22. Global Key Players of Medical Device Bioburden Testing, Product and Application
Table 23. Global Key Players of Medical Device Bioburden Testing, Date of Enter into This Industry
Table 24. Mergers & Acquisitions, Expansion Plans
Table 25. Global Medical Device Bioburden Testing Market Size by Type (2020-2025) & (US$ Million)
Table 26. Global Medical Device Bioburden Testing Revenue Market Share by Type (2020-2025)
Table 27. Global Medical Device Bioburden Testing Forecasted Market Size by Type (2026-2031) & (US$ Million)
Table 28. Global Medical Device Bioburden Testing Revenue Market Share by Type (2026-2031)
Table 29. Global Medical Device Bioburden Testing Market Size by Application (2020-2025) & (US$ Million)
Table 30. Global Medical Device Bioburden Testing Revenue Market Share by Application (2020-2025)
Table 31. Global Medical Device Bioburden Testing Forecasted Market Size by Application (2026-2031) & (US$ Million)
Table 32. Global Medical Device Bioburden Testing Revenue Market Share by Application (2026-2031)
Table 33. North America Medical Device Bioburden Testing Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 34. North America Medical Device Bioburden Testing Market Size by Country (2020-2025) & (US$ Million)
Table 35. North America Medical Device Bioburden Testing Market Size by Country (2026-2031) & (US$ Million)
Table 36. Europe Medical Device Bioburden Testing Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 37. Europe Medical Device Bioburden Testing Market Size by Country (2020-2025) & (US$ Million)
Table 38. Europe Medical Device Bioburden Testing Market Size by Country (2026-2031) & (US$ Million)
Table 39. Asia-Pacific Medical Device Bioburden Testing Market Size Growth Rate by Region (US$ Million): 2020 VS 2024 VS 2031
Table 40. Asia-Pacific Medical Device Bioburden Testing Market Size by Region (2020-2025) & (US$ Million)
Table 41. Asia-Pacific Medical Device Bioburden Testing Market Size by Region (2026-2031) & (US$ Million)
Table 42. Latin America Medical Device Bioburden Testing Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 43. Latin America Medical Device Bioburden Testing Market Size by Country (2020-2025) & (US$ Million)
Table 44. Latin America Medical Device Bioburden Testing Market Size by Country (2026-2031) & (US$ Million)
Table 45. Middle East & Africa Medical Device Bioburden Testing Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 46. Middle East & Africa Medical Device Bioburden Testing Market Size by Country (2020-2025) & (US$ Million)
Table 47. Middle East & Africa Medical Device Bioburden Testing Market Size by Country (2026-2031) & (US$ Million)
Table 48. Merck Company Details
Table 49. Merck Business Overview
Table 50. Merck Medical Device Bioburden Testing Product
Table 51. Merck Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 52. Merck Recent Development
Table 53. Eurofins Company Details
Table 54. Eurofins Business Overview
Table 55. Eurofins Medical Device Bioburden Testing Product
Table 56. Eurofins Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 57. Eurofins Recent Development
Table 58. TUV SUD Company Details
Table 59. TUV SUD Business Overview
Table 60. TUV SUD Medical Device Bioburden Testing Product
Table 61. TUV SUD Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 62. TUV SUD Recent Development
Table 63. Pacific BioLabs Company Details
Table 64. Pacific BioLabs Business Overview
Table 65. Pacific BioLabs Medical Device Bioburden Testing Product
Table 66. Pacific BioLabs Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 67. Pacific BioLabs Recent Development
Table 68. Nelson Labs Company Details
Table 69. Nelson Labs Business Overview
Table 70. Nelson Labs Medical Device Bioburden Testing Product
Table 71. Nelson Labs Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 72. Nelson Labs Recent Development
Table 73. SGS Company Details
Table 74. SGS Business Overview
Table 75. SGS Medical Device Bioburden Testing Product
Table 76. SGS Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 77. SGS Recent Development
Table 78. Charles River Laboratories Company Details
Table 79. Charles River Laboratories Business Overview
Table 80. Charles River Laboratories Medical Device Bioburden Testing Product
Table 81. Charles River Laboratories Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 82. Charles River Laboratories Recent Development
Table 83. Element Company Details
Table 84. Element Business Overview
Table 85. Element Medical Device Bioburden Testing Product
Table 86. Element Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 87. Element Recent Development
Table 88. WuXi AppTec Company Details
Table 89. WuXi AppTec Business Overview
Table 90. WuXi AppTec Medical Device Bioburden Testing Product
Table 91. WuXi AppTec Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 92. WuXi AppTec Recent Development
Table 93. Boston Analytical Company Details
Table 94. Boston Analytical Business Overview
Table 95. Boston Analytical Medical Device Bioburden Testing Product
Table 96. Boston Analytical Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 97. Boston Analytical Recent Development
Table 98. Ethide Laboratories Company Details
Table 99. Ethide Laboratories Business Overview
Table 100. Ethide Laboratories Medical Device Bioburden Testing Product
Table 101. Ethide Laboratories Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 102. Ethide Laboratories Recent Development
Table 103. Nova Biologicals Company Details
Table 104. Nova Biologicals Business Overview
Table 105. Nova Biologicals Medical Device Bioburden Testing Product
Table 106. Nova Biologicals Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 107. Nova Biologicals Recent Development
Table 108. Pace Analytical Company Details
Table 109. Pace Analytical Business Overview
Table 110. Pace Analytical Medical Device Bioburden Testing Product
Table 111. Pace Analytical Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 112. Pace Analytical Recent Development
Table 113. Wickham Laboratories Company Details
Table 114. Wickham Laboratories Business Overview
Table 115. Wickham Laboratories Medical Device Bioburden Testing Product
Table 116. Wickham Laboratories Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 117. Wickham Laboratories Recent Development
Table 118. FOCUS Laboratories Company Details
Table 119. FOCUS Laboratories Business Overview
Table 120. FOCUS Laboratories Medical Device Bioburden Testing Product
Table 121. FOCUS Laboratories Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 122. FOCUS Laboratories Recent Development
Table 123. UFAG Laboratorien AG Company Details
Table 124. UFAG Laboratorien AG Business Overview
Table 125. UFAG Laboratorien AG Medical Device Bioburden Testing Product
Table 126. UFAG Laboratorien AG Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 127. UFAG Laboratorien AG Recent Development
Table 128. SaniChem Resources Company Details
Table 129. SaniChem Resources Business Overview
Table 130. SaniChem Resources Medical Device Bioburden Testing Product
Table 131. SaniChem Resources Revenue in Medical Device Bioburden Testing Business (2020-2025) & (US$ Million)
Table 132. SaniChem Resources Recent Development
Table 133. Research Programs/Design for This Report
Table 134. Key Data Information from Secondary Sources
Table 135. Key Data Information from Primary Sources
Table 136. Authors List of This Report
List of Figures
Figure 1. Medical Device Bioburden Testing Picture
Figure 2. Global Medical Device Bioburden Testing Market Size Comparison by Type (2020-2031) & (US$ Million)
Figure 3. Global Medical Device Bioburden Testing Market Share by Type: 2024 VS 2031
Figure 4. Membrane Filtration Method Features
Figure 5. Most-Probable-Number Method Features
Figure 6. Pour-Plate Method Features
Figure 7. Surface-Spread Method Features
Figure 8. Global Medical Device Bioburden Testing Market Size by Application (2020-2031) & (US$ Million)
Figure 9. Global Medical Device Bioburden Testing Market Share by Application: 2024 VS 2031
Figure 10. Medical Equipment Case Studies
Figure 11. Medical Consumables Case Studies
Figure 12. Others Case Studies
Figure 13. Medical Device Bioburden Testing Report Years Considered
Figure 14. Global Medical Device Bioburden Testing Market Size (US$ Million), Year-over-Year: 2020-2031
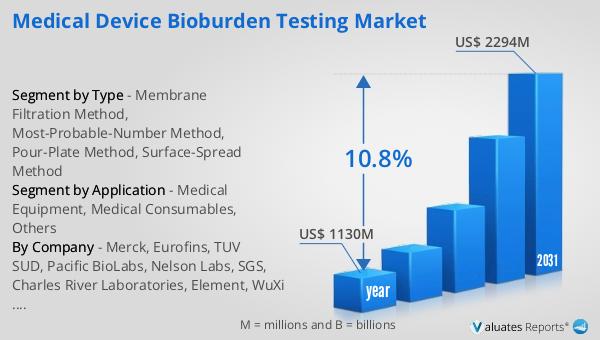
Figure 15. Global Medical Device Bioburden Testing Market Size, (US$ Million), 2020 VS 2024 VS 2031
Figure 16. Global Medical Device Bioburden Testing Market Share by Region: 2024 VS 2031
Figure 17. Global Medical Device Bioburden Testing Market Share by Players in 2024
Figure 18. Global Top Medical Device Bioburden Testing Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Medical Device Bioburden Testing as of 2024)
Figure 19. The Top 10 and 5 Players Market Share by Medical Device Bioburden Testing Revenue in 2024
Figure 20. North America Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 21. North America Medical Device Bioburden Testing Market Share by Country (2020-2031)
Figure 22. United States Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 23. Canada Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 24. Europe Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 25. Europe Medical Device Bioburden Testing Market Share by Country (2020-2031)
Figure 26. Germany Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 27. France Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 28. U.K. Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 29. Italy Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 30. Russia Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 31. Nordic Countries Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 32. Asia-Pacific Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 33. Asia-Pacific Medical Device Bioburden Testing Market Share by Region (2020-2031)
Figure 34. China Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 35. Japan Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 36. South Korea Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 37. Southeast Asia Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 38. India Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 39. Australia Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 40. Latin America Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 41. Latin America Medical Device Bioburden Testing Market Share by Country (2020-2031)
Figure 42. Mexico Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 43. Brazil Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 44. Middle East & Africa Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 45. Middle East & Africa Medical Device Bioburden Testing Market Share by Country (2020-2031)
Figure 46. Turkey Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 47. Saudi Arabia Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 48. UAE Medical Device Bioburden Testing Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 49. Merck Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 50. Eurofins Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 51. TUV SUD Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 52. Pacific BioLabs Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 53. Nelson Labs Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 54. SGS Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 55. Charles River Laboratories Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 56. Element Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 57. WuXi AppTec Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 58. Boston Analytical Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 59. Ethide Laboratories Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 60. Nova Biologicals Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 61. Pace Analytical Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 62. Wickham Laboratories Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 63. FOCUS Laboratories Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 64. UFAG Laboratorien AG Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 65. SaniChem Resources Revenue Growth Rate in Medical Device Bioburden Testing Business (2020-2025)
Figure 66. Bottom-up and Top-down Approaches for This Report
Figure 67. Data Triangulation
Figure 68. Key Executives Interviewed