List of Tables
Table 1. Global Biologics and Biosimilars CXO Services Market Size Growth Rate by Type (US$ Million): 2019 VS 2023 VS 2030
Table 2. Key Players of CRO
Table 3. Key Players of CDMO/CMO
Table 4. Key Players of CSO
Table 5. Global Biologics and Biosimilars CXO Services Market Size Growth by Application (US$ Million): 2019 VS 2023 VS 2030
Table 6. Global Biologics and Biosimilars CXO Services Market Size by Region (US$ Million): 2019 VS 2023 VS 2030
Table 7. Global Biologics and Biosimilars CXO Services Market Size by Region (2019-2024) & (US$ Million)
Table 8. Global Biologics and Biosimilars CXO Services Market Share by Region (2019-2024)
Table 9. Global Biologics and Biosimilars CXO Services Forecasted Market Size by Region (2025-2030) & (US$ Million)
Table 10. Global Biologics and Biosimilars CXO Services Market Share by Region (2025-2030)
Table 11. Biologics and Biosimilars CXO Services Market Trends
Table 12. Biologics and Biosimilars CXO Services Market Drivers
Table 13. Biologics and Biosimilars CXO Services Market Challenges
Table 14. Biologics and Biosimilars CXO Services Market Restraints
Table 15. Global Biologics and Biosimilars CXO Services Revenue by Players (2019-2024) & (US$ Million)
Table 16. Global Biologics and Biosimilars CXO Services Market Share by Players (2019-2024)
Table 17. Global Top Biologics and Biosimilars CXO Services Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Biologics and Biosimilars CXO Services as of 2023)
Table 18. Ranking of Global Top Biologics and Biosimilars CXO Services Companies by Revenue (US$ Million) in 2023
Table 19. Global 5 Largest Players Market Share by Biologics and Biosimilars CXO Services Revenue (CR5 and HHI) & (2019-2024)
Table 20. Global Key Players of Biologics and Biosimilars CXO Services, Headquarters and Area Served
Table 21. Global Key Players of Biologics and Biosimilars CXO Services, Product and Application
Table 22. Global Key Players of Biologics and Biosimilars CXO Services, Date of Enter into This Industry
Table 23. Mergers & Acquisitions, Expansion Plans
Table 24. Global Biologics and Biosimilars CXO Services Market Size by Type (2019-2024) & (US$ Million)
Table 25. Global Biologics and Biosimilars CXO Services Revenue Market Share by Type (2019-2024)
Table 26. Global Biologics and Biosimilars CXO Services Forecasted Market Size by Type (2025-2030) & (US$ Million)
Table 27. Global Biologics and Biosimilars CXO Services Revenue Market Share by Type (2025-2030)
Table 28. Global Biologics and Biosimilars CXO Services Market Size by Application (2019-2024) & (US$ Million)
Table 29. Global Biologics and Biosimilars CXO Services Revenue Market Share by Application (2019-2024)
Table 30. Global Biologics and Biosimilars CXO Services Forecasted Market Size by Application (2025-2030) & (US$ Million)
Table 31. Global Biologics and Biosimilars CXO Services Revenue Market Share by Application (2025-2030)
Table 32. North America Biologics and Biosimilars CXO Services Market Size Growth Rate by Country (US$ Million): 2019 VS 2023 VS 2030
Table 33. North America Biologics and Biosimilars CXO Services Market Size by Country (2019-2024) & (US$ Million)
Table 34. North America Biologics and Biosimilars CXO Services Market Size by Country (2025-2030) & (US$ Million)
Table 35. Europe Biologics and Biosimilars CXO Services Market Size Growth Rate by Country (US$ Million): 2019 VS 2023 VS 2030
Table 36. Europe Biologics and Biosimilars CXO Services Market Size by Country (2019-2024) & (US$ Million)
Table 37. Europe Biologics and Biosimilars CXO Services Market Size by Country (2025-2030) & (US$ Million)
Table 38. Asia-Pacific Biologics and Biosimilars CXO Services Market Size Growth Rate by Country (US$ Million): 2019 VS 2023 VS 2030
Table 39. Asia-Pacific Biologics and Biosimilars CXO Services Market Size by Region (2019-2024) & (US$ Million)
Table 40. Asia-Pacific Biologics and Biosimilars CXO Services Market Size by Region (2025-2030) & (US$ Million)
Table 41. Latin America Biologics and Biosimilars CXO Services Market Size Growth Rate by Country (US$ Million): 2019 VS 2023 VS 2030
Table 42. Latin America Biologics and Biosimilars CXO Services Market Size by Country (2019-2024) & (US$ Million)
Table 43. Latin America Biologics and Biosimilars CXO Services Market Size by Country (2025-2030) & (US$ Million)
Table 44. Middle East & Africa Biologics and Biosimilars CXO Services Market Size Growth Rate by Country (US$ Million): 2019 VS 2023 VS 2030
Table 45. Middle East & Africa Biologics and Biosimilars CXO Services Market Size by Country (2019-2024) & (US$ Million)
Table 46. Middle East & Africa Biologics and Biosimilars CXO Services Market Size by Country (2025-2030) & (US$ Million)
Table 47. Catalent Company Details
Table 48. Catalent Business Overview
Table 49. Catalent Biologics and Biosimilars CXO Services Product
Table 50. Catalent Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 51. Catalent Recent Development
Table 52. Thermo Fisher Scientific Company Details
Table 53. Thermo Fisher Scientific Business Overview
Table 54. Thermo Fisher Scientific Biologics and Biosimilars CXO Services Product
Table 55. Thermo Fisher Scientific Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 56. Thermo Fisher Scientific Recent Development
Table 57. Lonza Company Details
Table 58. Lonza Business Overview
Table 59. Lonza Biologics and Biosimilars CXO Services Product
Table 60. Lonza Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 61. Lonza Recent Development
Table 62. Siegfried Company Details
Table 63. Siegfried Business Overview
Table 64. Siegfried Biologics and Biosimilars CXO Services Product
Table 65. Siegfried Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 66. Siegfried Recent Development
Table 67. Recipharm Company Details
Table 68. Recipharm Business Overview
Table 69. Recipharm Biologics and Biosimilars CXO Services Product
Table 70. Recipharm Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 71. Recipharm Recent Development
Table 72. Boehringer Ingelheim Company Details
Table 73. Boehringer Ingelheim Business Overview
Table 74. Boehringer Ingelheim Biologics and Biosimilars CXO Services Product
Table 75. Boehringer Ingelheim Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 76. Boehringer Ingelheim Recent Development
Table 77. WuXi AppTech Company Details
Table 78. WuXi AppTech Business Overview
Table 79. WuXi AppTech Biologics and Biosimilars CXO Services Product
Table 80. WuXi AppTech Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 81. WuXi AppTech Recent Development
Table 82. WuXi Biologics Company Details
Table 83. WuXi Biologics Business Overview
Table 84. WuXi Biologics Biologics and Biosimilars CXO Services Product
Table 85. WuXi Biologics Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 86. WuXi Biologics Recent Development
Table 87. Fareva Company Details
Table 88. Fareva Business Overview
Table 89. Fareva Biologics and Biosimilars CXO Services Product
Table 90. Fareva Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 91. Fareva Recent Development
Table 92. Samsung Biologics Company Details
Table 93. Samsung Biologics Business Overview
Table 94. Samsung Biologics Biologics and Biosimilars CXO Services Product
Table 95. Samsung Biologics Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 96. Samsung Biologics Recent Development
Table 97. Aenova Company Details
Table 98. Aenova Business Overview
Table 99. Aenova Biologics and Biosimilars CXO Services Product
Table 100. Aenova Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 101. Aenova Recent Development
Table 102. Delpharm Company Details
Table 103. Delpharm Business Overview
Table 104. Delpharm Biologics and Biosimilars CXO Services Product
Table 105. Delpharm Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 106. Delpharm Recent Development
Table 107. Strides Pharma Company Details
Table 108. Strides Pharma Business Overview
Table 109. Strides Pharma Biologics and Biosimilars CXO Services Product
Table 110. Strides Pharma Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 111. Strides Pharma Recent Development
Table 112. Piramal Company Details
Table 113. Piramal Business Overview
Table 114. Piramal Biologics and Biosimilars CXO Services Product
Table 115. Piramal Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 116. Piramal Recent Development
Table 117. Famar Company Details
Table 118. Famar Business Overview
Table 119. Famar Biologics and Biosimilars CXO Services Product
Table 120. Famar Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 121. Famar Recent Development
Table 122. Curia Company Details
Table 123. Curia Business Overview
Table 124. Curia Biologics and Biosimilars CXO Services Product
Table 125. Curia Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 126. Curia Recent Development
Table 127. Jubilant Company Details
Table 128. Jubilant Business Overview
Table 129. Jubilant Biologics and Biosimilars CXO Services Product
Table 130. Jubilant Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 131. Jubilant Recent Development
Table 132. Vetter Company Details
Table 133. Vetter Business Overview
Table 134. Vetter Biologics and Biosimilars CXO Services Product
Table 135. Vetter Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 136. Vetter Recent Development
Table 137. AGC Pharma Chemicals Company Details
Table 138. AGC Pharma Chemicals Business Overview
Table 139. AGC Pharma Chemicals Biologics and Biosimilars CXO Services Product
Table 140. AGC Pharma Chemicals Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 141. AGC Pharma Chemicals Recent Development
Table 142. Asymchem Company Details
Table 143. Asymchem Business Overview
Table 144. Asymchem Biologics and Biosimilars CXO Services Product
Table 145. Asymchem Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 146. Asymchem Recent Development
Table 147. Pharmaron Company Details
Table 148. Pharmaron Business Overview
Table 149. Pharmaron Biologics and Biosimilars CXO Services Product
Table 150. Pharmaron Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 151. Pharmaron Recent Development
Table 152. Eurofins Company Details
Table 153. Eurofins Business Overview
Table 154. Eurofins Biologics and Biosimilars CXO Services Product
Table 155. Eurofins Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 156. Eurofins Recent Development
Table 157. Ascendia Pharmaceuticals Company Details
Table 158. Ascendia Pharmaceuticals Business Overview
Table 159. Ascendia Pharmaceuticals Biologics and Biosimilars CXO Services Product
Table 160. Ascendia Pharmaceuticals Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 161. Ascendia Pharmaceuticals Recent Development
Table 162. Ardena Company Details
Table 163. Ardena Business Overview
Table 164. Ardena Biologics and Biosimilars CXO Services Product
Table 165. Ardena Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 166. Ardena Recent Development
Table 167. CPL Company Details
Table 168. CPL Business Overview
Table 169. CPL Biologics and Biosimilars CXO Services Product
Table 170. CPL Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 171. CPL Recent Development
Table 172. UPM Pharmaceuticals Company Details
Table 173. UPM Pharmaceuticals Business Overview
Table 174. UPM Pharmaceuticals Biologics and Biosimilars CXO Services Product
Table 175. UPM Pharmaceuticals Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 176. UPM Pharmaceuticals Recent Development
Table 177. FUJIFILM Diosynth Biotechnologies Company Details
Table 178. FUJIFILM Diosynth Biotechnologies Business Overview
Table 179. FUJIFILM Diosynth Biotechnologies Biologics and Biosimilars CXO Services Product
Table 180. FUJIFILM Diosynth Biotechnologies Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 181. FUJIFILM Diosynth Biotechnologies Recent Development
Table 182. Groupe Parima Company Details
Table 183. Groupe Parima Business Overview
Table 184. Groupe Parima Biologics and Biosimilars CXO Services Product
Table 185. Groupe Parima Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 186. Groupe Parima Recent Development
Table 187. TBD Pharmatech Company Details
Table 188. TBD Pharmatech Business Overview
Table 189. TBD Pharmatech Biologics and Biosimilars CXO Services Product
Table 190. TBD Pharmatech Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 191. TBD Pharmatech Recent Development
Table 192. Avid Bioservices Company Details
Table 193. Avid Bioservices Business Overview
Table 194. Avid Bioservices Biologics and Biosimilars CXO Services Product
Table 195. Avid Bioservices Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 196. Avid Bioservices Recent Development
Table 197. NextPharma Company Details
Table 198. NextPharma Business Overview
Table 199. NextPharma Biologics and Biosimilars CXO Services Product
Table 200. NextPharma Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 201. NextPharma Recent Development
Table 202. Alcami Company Details
Table 203. Alcami Business Overview
Table 204. Alcami Biologics and Biosimilars CXO Services Product
Table 205. Alcami Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 206. Alcami Recent Development
Table 207. Societal CDMO Company Details
Table 208. Societal CDMO Business Overview
Table 209. Societal CDMO Biologics and Biosimilars CXO Services Product
Table 210. Societal CDMO Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 211. Societal CDMO Recent Development
Table 212. MedPharm Company Details
Table 213. MedPharm Business Overview
Table 214. MedPharm Biologics and Biosimilars CXO Services Product
Table 215. MedPharm Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 216. MedPharm Recent Development
Table 217. Euroapi Company Details
Table 218. Euroapi Business Overview
Table 219. Euroapi Biologics and Biosimilars CXO Services Product
Table 220. Euroapi Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 221. Euroapi Recent Development
Table 222. BioVectra Company Details
Table 223. BioVectra Business Overview
Table 224. BioVectra Biologics and Biosimilars CXO Services Product
Table 225. BioVectra Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 226. BioVectra Recent Development
Table 227. Pfizer CentreOne Company Details
Table 228. Pfizer CentreOne Business Overview
Table 229. Pfizer CentreOne Biologics and Biosimilars CXO Services Product
Table 230. Pfizer CentreOne Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 231. Pfizer CentreOne Recent Development
Table 232. Fermion Company Details
Table 233. Fermion Business Overview
Table 234. Fermion Biologics and Biosimilars CXO Services Product
Table 235. Fermion Revenue in Biologics and Biosimilars CXO Services Business (2019-2024) & (US$ Million)
Table 236. Fermion Recent Development
Table 237. Research Programs/Design for This Report
Table 238. Key Data Information from Secondary Sources
Table 239. Key Data Information from Primary Sources
Table 240. Authors List of This Report
List of Figures
Figure 1. Biologics and Biosimilars CXO Services Picture
Figure 2. Global Biologics and Biosimilars CXO Services Market Size Comparison by Type (2024-2030) & (US$ Million)
Figure 3. Global Biologics and Biosimilars CXO Services Market Share by Type: 2023 VS 2030
Figure 4. CRO Features
Figure 5. CDMO/CMO Features
Figure 6. CSO Features
Figure 7. Global Biologics and Biosimilars CXO Services Market Size by Application (2024-2030) & (US$ Million)
Figure 8. Global Biologics and Biosimilars CXO Services Market Share by Application: 2023 VS 2030
Figure 9. API CXO Case Studies
Figure 10. FDF CXO Case Studies
Figure 11. Other Case Studies
Figure 12. Biologics and Biosimilars CXO Services Report Years Considered
Figure 13. Global Biologics and Biosimilars CXO Services Market Size (US$ Million), Year-over-Year: 2019-2030
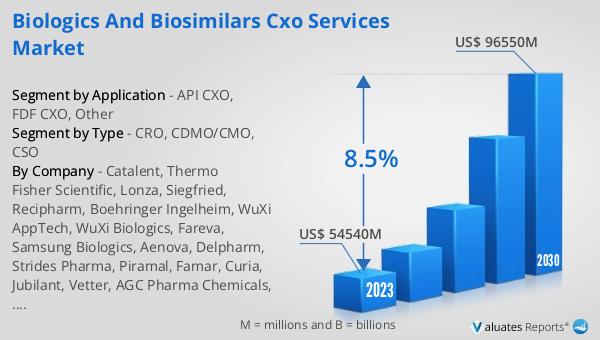
Figure 14. Global Biologics and Biosimilars CXO Services Market Size, (US$ Million), 2019 VS 2023 VS 2030
Figure 15. Global Biologics and Biosimilars CXO Services Market Share by Region: 2023 VS 2030
Figure 16. Global Biologics and Biosimilars CXO Services Market Share by Players in 2023
Figure 17. Global Top Biologics and Biosimilars CXO Services Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Biologics and Biosimilars CXO Services as of 2023)
Figure 18. The Top 10 and 5 Players Market Share by Biologics and Biosimilars CXO Services Revenue in 2023
Figure 19. North America Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 20. North America Biologics and Biosimilars CXO Services Market Share by Country (2019-2030)
Figure 21. United States Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 22. Canada Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 23. Europe Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 24. Europe Biologics and Biosimilars CXO Services Market Share by Country (2019-2030)
Figure 25. Germany Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 26. France Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 27. U.K. Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 28. Italy Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 29. Russia Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 30. Nordic Countries Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 31. Asia-Pacific Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 32. Asia-Pacific Biologics and Biosimilars CXO Services Market Share by Region (2019-2030)
Figure 33. China Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 34. Japan Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 35. South Korea Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 36. Southeast Asia Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 37. India Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 38. Australia Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 39. Latin America Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 40. Latin America Biologics and Biosimilars CXO Services Market Share by Country (2019-2030)
Figure 41. Mexico Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 42. Brazil Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 43. Middle East & Africa Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 44. Middle East & Africa Biologics and Biosimilars CXO Services Market Share by Country (2019-2030)
Figure 45. Turkey Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 46. Saudi Arabia Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 47. UAE Biologics and Biosimilars CXO Services Market Size YoY Growth (2019-2030) & (US$ Million)
Figure 48. Catalent Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 49. Thermo Fisher Scientific Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 50. Lonza Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 51. Siegfried Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 52. Recipharm Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 53. Boehringer Ingelheim Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 54. WuXi AppTech Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 55. WuXi Biologics Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 56. Fareva Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 57. Samsung Biologics Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 58. Aenova Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 59. Delpharm Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 60. Strides Pharma Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 61. Piramal Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 62. Famar Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 63. Curia Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 64. Jubilant Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 65. Vetter Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 66. AGC Pharma Chemicals Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 67. Asymchem Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 68. Pharmaron Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 69. Eurofins Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 70. Ascendia Pharmaceuticals Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 71. Ardena Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 72. CPL Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 73. UPM Pharmaceuticals Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 74. FUJIFILM Diosynth Biotechnologies Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 75. Groupe Parima Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 76. TBD Pharmatech Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 77. Avid Bioservices Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 78. NextPharma Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 79. Alcami Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 80. Societal CDMO Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 81. MedPharm Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 82. Euroapi Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 83. BioVectra Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 84. Pfizer CentreOne Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 85. Fermion Revenue Growth Rate in Biologics and Biosimilars CXO Services Business (2019-2024)
Figure 86. Bottom-up and Top-down Approaches for This Report
Figure 87. Data Triangulation
Figure 88. Key Executives Interviewed