List of Tables
Table 1. Medical Device Testing, Inspection, and Certification Market Trends
Table 2. Medical Device Testing, Inspection, and Certification Market Drivers & Opportunity
Table 3. Medical Device Testing, Inspection, and Certification Market Challenges
Table 4. Medical Device Testing, Inspection, and Certification Market Restraints
Table 5. Global Medical Device Testing, Inspection, and Certification Revenue by Company (2019-2024) & (US$ Million)
Table 6. Global Medical Device Testing, Inspection, and Certification Revenue Market Share by Company (2019-2024)
Table 7. Key Companies Medical Device Testing, Inspection, and Certification Manufacturing Base Distribution and Headquarters
Table 8. Key Companies Medical Device Testing, Inspection, and Certification Product Type
Table 9. Key Companies Time to Begin Mass Production of Medical Device Testing, Inspection, and Certification
Table 10. Global Medical Device Testing, Inspection, and Certification Companies Market Concentration Ratio (CR5 and HHI)
Table 11. Global Top Companies Market Share by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Medical Device Testing, Inspection, and Certification as of 2023)
Table 12. Mergers & Acquisitions, Expansion Plans
Table 13. Global Medical Device Testing, Inspection, and Certification Sales Value by Type: 2019 VS 2023 VS 2030 (US$ Million)
Table 14. Global Medical Device Testing, Inspection, and Certification Sales Value by Type (2019-2024) & (US$ Million)
Table 15. Global Medical Device Testing, Inspection, and Certification Sales Value by Type (2025-2030) & (US$ Million)
Table 16. Global Medical Device Testing, Inspection, and Certification Sales Market Share in Value by Type (2019-2024) & (%)
Table 17. Global Medical Device Testing, Inspection, and Certification Sales Market Share in Value by Type (2025-2030) & (%)
Table 18. Global Medical Device Testing, Inspection, and Certification Sales Value by Application: 2019 VS 2023 VS 2030 (US$ Million)
Table 19. Global Medical Device Testing, Inspection, and Certification Sales Value by Application (2019-2024) & (US$ Million)
Table 20. Global Medical Device Testing, Inspection, and Certification Sales Value by Application (2025-2030) & (US$ Million)
Table 21. Global Medical Device Testing, Inspection, and Certification Sales Market Share in Value by Application (2019-2024) & (%)
Table 22. Global Medical Device Testing, Inspection, and Certification Sales Market Share in Value by Application (2025-2030) & (%)
Table 23. Global Medical Device Testing, Inspection, and Certification Sales Value by Region: 2019 VS 2023 VS 2030 (US$ Million)
Table 24. Global Medical Device Testing, Inspection, and Certification Sales Value by Region (2019-2024) & (US$ Million)
Table 25. Global Medical Device Testing, Inspection, and Certification Sales Value by Region (2025-2030) & (US$ Million)
Table 26. Global Medical Device Testing, Inspection, and Certification Sales Value by Region (2019-2024) & (%)
Table 27. Global Medical Device Testing, Inspection, and Certification Sales Value by Region (2025-2030) & (%)
Table 28. Key Countries/Regions Medical Device Testing, Inspection, and Certification Sales Value Growth Trends, (US$ Million): 2019 VS 2023 VS 2030
Table 29. Key Countries/Regions Medical Device Testing, Inspection, and Certification Sales Value, (2019-2024) & (US$ Million)
Table 30. Key Countries/Regions Medical Device Testing, Inspection, and Certification Sales Value, (2025-2030) & (US$ Million)
Table 31. SGS Group Basic Information List
Table 32. SGS Group Description and Business Overview
Table 33. SGS Group Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 34. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of SGS Group (2019-2024)
Table 35. SGS Group Recent Developments
Table 36. Element Materials Technology Group Basic Information List
Table 37. Element Materials Technology Group Description and Business Overview
Table 38. Element Materials Technology Group Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 39. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Element Materials Technology Group (2019-2024)
Table 40. Element Materials Technology Group Recent Developments
Table 41. Intertek Basic Information List
Table 42. Intertek Description and Business Overview
Table 43. Intertek Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 44. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Intertek (2019-2024)
Table 45. Intertek Recent Developments
Table 46. Dekra Certification Basic Information List
Table 47. Dekra Certification Description and Business Overview
Table 48. Dekra Certification Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 49. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Dekra Certification (2019-2024)
Table 50. Dekra Certification Recent Developments
Table 51. TUV SUD Basic Information List
Table 52. TUV SUD Description and Business Overview
Table 53. TUV SUD Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 54. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of TUV SUD (2019-2024)
Table 55. TUV SUD Recent Developments
Table 56. UL LLC Basic Information List
Table 57. UL LLC Description and Business Overview
Table 58. UL LLC Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 59. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of UL LLC (2019-2024)
Table 60. UL LLC Recent Developments
Table 61. TUV Rheinland Basic Information List
Table 62. TUV Rheinland Description and Business Overview
Table 63. TUV Rheinland Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 64. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of TUV Rheinland (2019-2024)
Table 65. TUV Rheinland Recent Developments
Table 66. Merieux NutriSciences Basic Information List
Table 67. Merieux NutriSciences Description and Business Overview
Table 68. Merieux NutriSciences Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 69. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Merieux NutriSciences (2019-2024)
Table 70. Merieux NutriSciences Recent Developments
Table 71. F2 Labs Basic Information List
Table 72. F2 Labs Description and Business Overview
Table 73. F2 Labs Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 74. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of F2 Labs (2019-2024)
Table 75. F2 Labs Recent Developments
Table 76. Eurofins Scientific Basic Information List
Table 77. Eurofins Scientific Description and Business Overview
Table 78. Eurofins Scientific Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 79. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Eurofins Scientific (2019-2024)
Table 80. Eurofins Scientific Recent Developments
Table 81. Freyr Solutions Basic Information List
Table 82. Freyr Solutions Description and Business Overview
Table 83. Freyr Solutions Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 84. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Freyr Solutions (2019-2024)
Table 85. Freyr Solutions Recent Developments
Table 86. Smithers Basic Information List
Table 87. Smithers Description and Business Overview
Table 88. Smithers Medical Device Testing, Inspection, and Certification Products, Services and Solutions
Table 89. Revenue (US$ Million) in Medical Device Testing, Inspection, and Certification Business of Smithers (2019-2024)
Table 90. Smithers Recent Developments
Table 91. Key Raw Materials Lists
Table 92. Raw Materials Key Suppliers Lists
Table 93. Medical Device Testing, Inspection, and Certification Downstream Customers
Table 94. Medical Device Testing, Inspection, and Certification Distributors List
Table 95. Research Programs/Design for This Report
Table 96. Key Data Information from Secondary Sources
Table 97. Key Data Information from Primary Sources
Table 98. Business Unit and Senior & Team Lead Analysts
List of Figures
Figure 1. Medical Device Testing, Inspection, and Certification Product Picture
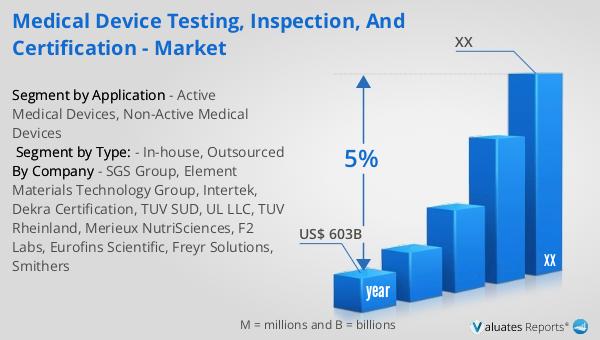
Figure 2. Global Medical Device Testing, Inspection, and Certification Sales Value, 2019 VS 2023 VS 2030 (US$ Million)
Figure 3. Global Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 4. Medical Device Testing, Inspection, and Certification Report Years Considered
Figure 5. Global Medical Device Testing, Inspection, and Certification Players Revenue Ranking (2023) & (US$ Million)
Figure 6. The 5 and 10 Largest Manufacturers in the World: Market Share by Medical Device Testing, Inspection, and Certification Revenue in 2023
Figure 7. Medical Device Testing, Inspection, and Certification Market Share by Company Type (Tier 1, Tier 2, and Tier 3): 2019 VS 2023
Figure 8. In-house Picture
Figure 9. Outsourced Picture
Figure 10. Global Medical Device Testing, Inspection, and Certification Sales Value by Type (2019 VS 2023 VS 2030) & (US$ Million)
Figure 11. Global Medical Device Testing, Inspection, and Certification Sales Value Market Share by Type, 2023 & 2030
Figure 12. Product Picture of Active Medical Devices
Figure 13. Product Picture of Non-Active Medical Devices
Figure 14. Global Medical Device Testing, Inspection, and Certification Sales Value by Application (2019 VS 2023 VS 2030) & (US$ Million)
Figure 15. Global Medical Device Testing, Inspection, and Certification Sales Value Market Share by Application, 2023 & 2030
Figure 16. North America Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 17. North America Medical Device Testing, Inspection, and Certification Sales Value by Country (%), 2023 VS 2030
Figure 18. Europe Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 19. Europe Medical Device Testing, Inspection, and Certification Sales Value by Country (%), 2023 VS 2030
Figure 20. Asia Pacific Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 21. Asia Pacific Medical Device Testing, Inspection, and Certification Sales Value by Country (%), 2023 VS 2030
Figure 22. South America Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 23. South America Medical Device Testing, Inspection, and Certification Sales Value by Country (%), 2023 VS 2030
Figure 24. Middle East & Africa Medical Device Testing, Inspection, and Certification Sales Value (2019-2030) & (US$ Million)
Figure 25. Middle East & Africa Medical Device Testing, Inspection, and Certification Sales Value by Country (%), 2023 VS 2030
Figure 26. Key Countries/Regions Medical Device Testing, Inspection, and Certification Sales Value (%), (2019-2030)
Figure 27. United States Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 28. United States Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 29. United States Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 30. Europe Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 31. Europe Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 32. Europe Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 33. China Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 34. China Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 35. China Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 36. Japan Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 37. Japan Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 38. Japan Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 39. South Korea Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 40. South Korea Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 41. South Korea Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 42. Southeast Asia Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 43. Southeast Asia Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 44. Southeast Asia Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 45. India Medical Device Testing, Inspection, and Certification Sales Value, (2019-2030) & (US$ Million)
Figure 46. India Medical Device Testing, Inspection, and Certification Sales Value by Type (%), 2023 VS 2030
Figure 47. India Medical Device Testing, Inspection, and Certification Sales Value by Application (%), 2023 VS 2030
Figure 48. Medical Device Testing, Inspection, and Certification Industrial Chain
Figure 49. Medical Device Testing, Inspection, and Certification Manufacturing Cost Structure
Figure 50. Channels of Distribution (Direct Sales, and Distribution)
Figure 51. Bottom-up and Top-down Approaches for This Report
Figure 52. Data Triangulation