List of Tables
Table 1. In-vitro Diagnostic Services Market Trends
Table 2. In-vitro Diagnostic Services Market Drivers & Opportunity
Table 3. In-vitro Diagnostic Services Market Challenges
Table 4. In-vitro Diagnostic Services Market Restraints
Table 5. Global In-vitro Diagnostic Services Revenue by Company (2019-2024) & (US$ Million)
Table 6. Global In-vitro Diagnostic Services Revenue Market Share by Company (2019-2024)
Table 7. Key Companies In-vitro Diagnostic Services Manufacturing Base Distribution and Headquarters
Table 8. Key Companies In-vitro Diagnostic Services Product Type
Table 9. Key Companies Time to Begin Mass Production of In-vitro Diagnostic Services
Table 10. Global In-vitro Diagnostic Services Companies Market Concentration Ratio (CR5 and HHI)
Table 11. Global Top Companies Market Share by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in In-vitro Diagnostic Services as of 2023)
Table 12. Mergers & Acquisitions, Expansion Plans
Table 13. Global In-vitro Diagnostic Services Sales Value by Type: 2019 VS 2023 VS 2030 (US$ Million)
Table 14. Global In-vitro Diagnostic Services Sales Value by Type (2019-2024) & (US$ Million)
Table 15. Global In-vitro Diagnostic Services Sales Value by Type (2025-2030) & (US$ Million)
Table 16. Global In-vitro Diagnostic Services Sales Market Share in Value by Type (2019-2024) & (%)
Table 17. Global In-vitro Diagnostic Services Sales Market Share in Value by Type (2025-2030) & (%)
Table 18. Global In-vitro Diagnostic Services Sales Value by Application: 2019 VS 2023 VS 2030 (US$ Million)
Table 19. Global In-vitro Diagnostic Services Sales Value by Application (2019-2024) & (US$ Million)
Table 20. Global In-vitro Diagnostic Services Sales Value by Application (2025-2030) & (US$ Million)
Table 21. Global In-vitro Diagnostic Services Sales Market Share in Value by Application (2019-2024) & (%)
Table 22. Global In-vitro Diagnostic Services Sales Market Share in Value by Application (2025-2030) & (%)
Table 23. Global In-vitro Diagnostic Services Sales Value by Region: 2019 VS 2023 VS 2030 (US$ Million)
Table 24. Global In-vitro Diagnostic Services Sales Value by Region (2019-2024) & (US$ Million)
Table 25. Global In-vitro Diagnostic Services Sales Value by Region (2025-2030) & (US$ Million)
Table 26. Global In-vitro Diagnostic Services Sales Value by Region (2019-2024) & (%)
Table 27. Global In-vitro Diagnostic Services Sales Value by Region (2025-2030) & (%)
Table 28. Key Countries/Regions In-vitro Diagnostic Services Sales Value Growth Trends, (US$ Million): 2019 VS 2023 VS 2030
Table 29. Key Countries/Regions In-vitro Diagnostic Services Sales Value, (2019-2024) & (US$ Million)
Table 30. Key Countries/Regions In-vitro Diagnostic Services Sales Value, (2025-2030) & (US$ Million)
Table 31. BioRad Basic Information List
Table 32. BioRad Description and Business Overview
Table 33. BioRad In-vitro Diagnostic Services Products, Services and Solutions
Table 34. Revenue (US$ Million) in In-vitro Diagnostic Services Business of BioRad (2019-2024)
Table 35. BioRad Recent Developments
Table 36. DexCom Basic Information List
Table 37. DexCom Description and Business Overview
Table 38. DexCom In-vitro Diagnostic Services Products, Services and Solutions
Table 39. Revenue (US$ Million) in In-vitro Diagnostic Services Business of DexCom (2019-2024)
Table 40. DexCom Recent Developments
Table 41. Nova Biomedical Basic Information List
Table 42. Nova Biomedical Description and Business Overview
Table 43. Nova Biomedical In-vitro Diagnostic Services Products, Services and Solutions
Table 44. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Nova Biomedical (2019-2024)
Table 45. Nova Biomedical Recent Developments
Table 46. OraSure Basic Information List
Table 47. OraSure Description and Business Overview
Table 48. OraSure In-vitro Diagnostic Services Products, Services and Solutions
Table 49. Revenue (US$ Million) in In-vitro Diagnostic Services Business of OraSure (2019-2024)
Table 50. OraSure Recent Developments
Table 51. Pearl Pathways Basic Information List
Table 52. Pearl Pathways Description and Business Overview
Table 53. Pearl Pathways In-vitro Diagnostic Services Products, Services and Solutions
Table 54. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Pearl Pathways (2019-2024)
Table 55. Pearl Pathways Recent Developments
Table 56. Phenomenex Basic Information List
Table 57. Phenomenex Description and Business Overview
Table 58. Phenomenex In-vitro Diagnostic Services Products, Services and Solutions
Table 59. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Phenomenex (2019-2024)
Table 60. Phenomenex Recent Developments
Table 61. Qiagen Basic Information List
Table 62. Qiagen Description and Business Overview
Table 63. Qiagen In-vitro Diagnostic Services Products, Services and Solutions
Table 64. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Qiagen (2019-2024)
Table 65. Qiagen Recent Developments
Table 66. Roche Diagnostics Basic Information List
Table 67. Roche Diagnostics Description and Business Overview
Table 68. Roche Diagnostics In-vitro Diagnostic Services Products, Services and Solutions
Table 69. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Roche Diagnostics (2019-2024)
Table 70. Roche Diagnostics Recent Developments
Table 71. Siemens Basic Information List
Table 72. Siemens Description and Business Overview
Table 73. Siemens In-vitro Diagnostic Services Products, Services and Solutions
Table 74. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Siemens (2019-2024)
Table 75. Siemens Recent Developments
Table 76. Sysmex Basic Information List
Table 77. Sysmex Description and Business Overview
Table 78. Sysmex In-vitro Diagnostic Services Products, Services and Solutions
Table 79. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Sysmex (2019-2024)
Table 80. Sysmex Recent Developments
Table 81. Thermofisher Basic Information List
Table 82. Thermofisher Description and Business Overview
Table 83. Thermofisher In-vitro Diagnostic Services Products, Services and Solutions
Table 84. Revenue (US$ Million) in In-vitro Diagnostic Services Business of Thermofisher (2019-2024)
Table 85. Thermofisher Recent Developments
Table 86. Key Raw Materials Lists
Table 87. Raw Materials Key Suppliers Lists
Table 88. In-vitro Diagnostic Services Downstream Customers
Table 89. In-vitro Diagnostic Services Distributors List
Table 90. Research Programs/Design for This Report
Table 91. Key Data Information from Secondary Sources
Table 92. Key Data Information from Primary Sources
Table 93. Business Unit and Senior & Team Lead Analysts
List of Figures
Figure 1. In-vitro Diagnostic Services Product Picture
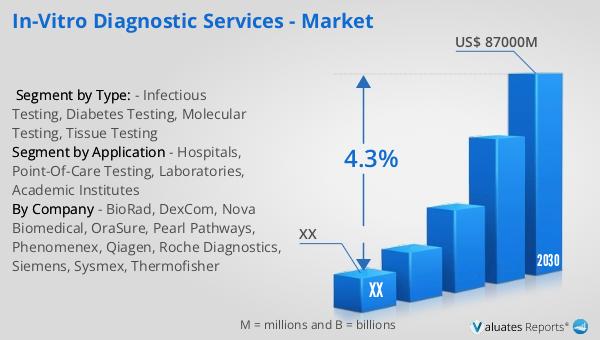
Figure 2. Global In-vitro Diagnostic Services Sales Value, 2019 VS 2023 VS 2030 (US$ Million)
Figure 3. Global In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 4. In-vitro Diagnostic Services Report Years Considered
Figure 5. Global In-vitro Diagnostic Services Players Revenue Ranking (2023) & (US$ Million)
Figure 6. The 5 and 10 Largest Manufacturers in the World: Market Share by In-vitro Diagnostic Services Revenue in 2023
Figure 7. In-vitro Diagnostic Services Market Share by Company Type (Tier 1, Tier 2, and Tier 3): 2019 VS 2023
Figure 8. Infectious Testing Picture
Figure 9. Diabetes Testing Picture
Figure 10. Molecular Testing Picture
Figure 11. Tissue Testing Picture
Figure 12. Global In-vitro Diagnostic Services Sales Value by Type (2019 VS 2023 VS 2030) & (US$ Million)
Figure 13. Global In-vitro Diagnostic Services Sales Value Market Share by Type, 2023 & 2030
Figure 14. Product Picture of Hospitals
Figure 15. Product Picture of Point-Of-Care Testing
Figure 16. Product Picture of Laboratories
Figure 17. Product Picture of Academic Institutes
Figure 18. Global In-vitro Diagnostic Services Sales Value by Application (2019 VS 2023 VS 2030) & (US$ Million)
Figure 19. Global In-vitro Diagnostic Services Sales Value Market Share by Application, 2023 & 2030
Figure 20. North America In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 21. North America In-vitro Diagnostic Services Sales Value by Country (%), 2023 VS 2030
Figure 22. Europe In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 23. Europe In-vitro Diagnostic Services Sales Value by Country (%), 2023 VS 2030
Figure 24. Asia Pacific In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 25. Asia Pacific In-vitro Diagnostic Services Sales Value by Country (%), 2023 VS 2030
Figure 26. South America In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 27. South America In-vitro Diagnostic Services Sales Value by Country (%), 2023 VS 2030
Figure 28. Middle East & Africa In-vitro Diagnostic Services Sales Value (2019-2030) & (US$ Million)
Figure 29. Middle East & Africa In-vitro Diagnostic Services Sales Value by Country (%), 2023 VS 2030
Figure 30. Key Countries/Regions In-vitro Diagnostic Services Sales Value (%), (2019-2030)
Figure 31. United States In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 32. United States In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 33. United States In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 34. Europe In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 35. Europe In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 36. Europe In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 37. China In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 38. China In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 39. China In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 40. Japan In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 41. Japan In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 42. Japan In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 43. South Korea In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 44. South Korea In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 45. South Korea In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 46. Southeast Asia In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 47. Southeast Asia In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 48. Southeast Asia In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 49. India In-vitro Diagnostic Services Sales Value, (2019-2030) & (US$ Million)
Figure 50. India In-vitro Diagnostic Services Sales Value by Type (%), 2023 VS 2030
Figure 51. India In-vitro Diagnostic Services Sales Value by Application (%), 2023 VS 2030
Figure 52. In-vitro Diagnostic Services Industrial Chain
Figure 53. In-vitro Diagnostic Services Manufacturing Cost Structure
Figure 54. Channels of Distribution (Direct Sales, and Distribution)
Figure 55. Bottom-up and Top-down Approaches for This Report
Figure 56. Data Triangulation