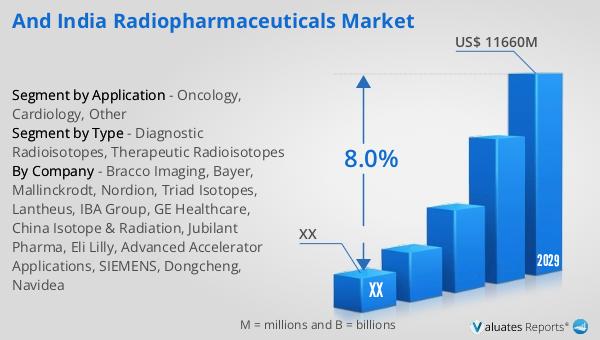
Ans: The and India Radiopharmaceuticals Market size in 2029 will be US$ 11660 million.

The global Radiopharmaceuticals revenue was US$ 6761 million in 2022 and is forecast to a readjusted size of US$ 11660 million by 2029 with a CAGR of 8.0% during the forecast period (2023-2029).
Regulatory Frameworks and Nuclear Medicine Advancement
National nuclear regulatory authorities have established comprehensive oversight frameworks governing radiopharmaceutical production, distribution, and clinical application. Healthcare regulatory agencies are developing expedited approval pathways for diagnostic and therapeutic radiopharmaceuticals addressing unmet medical needs. Pharmacopoeial authorities are creating standards for radiopharmaceutical quality, purity, and sterility specific to short-lived radioactive materials. International atomic energy organizations provide guidance on facility design, operational safety, and quality management for radiopharmaceutical manufacturing.
Government healthcare programs are expanding reimbursement coverage for nuclear medicine procedures demonstrating clinical value and cost-effectiveness. Public health initiatives addressing cancer early detection emphasize the role of molecular imaging in screening programs. Research funding agencies are prioritizing theranostic approaches that combine diagnostic imaging with targeted radionuclide therapy.
Indian regulatory authorities have streamlined approval processes for radiopharmaceutical facilities and products to support growing healthcare infrastructure. National atomic energy programs are facilitating isotope production capabilities and distribution networks. Healthcare policy frameworks increasingly recognize nuclear medicine as essential to comprehensive cancer care programs.
Precision Medicine and Theranostic Approaches
Oncology practice is undergoing transformation toward molecularly targeted therapies guided by diagnostic imaging. Theranostic radiopharmaceuticals enable personalized treatment selection based on individual tumor characteristics visualized through molecular imaging. Companion diagnostic concepts are being applied to radiopharmaceuticals, where imaging agents predict therapeutic response to corresponding radionuclide therapies. Genomic profiling integration with molecular imaging provides comprehensive tumor characterization guiding treatment decisions.
Clinical trial design is incorporating imaging biomarkers as endpoints for therapeutic efficacy assessment. Pharmaceutical companies are developing targeted radiotherapeutics addressing specific molecular pathways in cancer cells. Patient stratification based on radiopharmaceutical imaging improves clinical trial efficiency and therapeutic outcome prediction.
Isotope Production and Supply Chain Development
Global initiatives are addressing medical isotope supply security through diverse production methods and geographic distribution. Accelerator-based isotope production technologies are emerging as alternatives to reactor-based production. Domestic isotope production capabilities are developing in regions previously dependent on international supply. Public-private partnerships are facilitating investment in isotope production infrastructure.
Short-lived isotope applications are driving development of regional production and distribution networks minimizing decay-related losses. Isotope generator systems enable point-of-use radiopharmaceutical preparation in healthcare facilities. Supply chain resilience has become strategic priority following historical isotope shortage episodes that disrupted clinical services.
Technological Innovation in Imaging and Therapy
Hybrid imaging modalities combining positron emission tomography with computed tomography or magnetic resonance imaging enable precise anatomical localization of molecular information. Detector technology advances have improved imaging sensitivity and spatial resolution. Image reconstruction algorithms incorporating artificial intelligence enhance image quality while reducing radiation exposure. Total-body positron emission tomography systems enable novel research applications and potentially more sensitive disease detection.
Alpha-particle emitting radiopharmaceuticals represent emerging therapeutic modality with potential advantages over beta-emitting alternatives. Targeted radionuclide therapy is expanding beyond traditional applications into new disease areas. Dosimetry methodologies are becoming more sophisticated, enabling individualized treatment planning.
Clinical Evidence Development and Practice Integration
Clinical research is generating robust evidence supporting radiopharmaceutical applications across diverse medical conditions. Practice guidelines from medical specialty societies are incorporating nuclear medicine procedures into diagnostic and treatment algorithms. Multidisciplinary tumor boards increasingly utilize molecular imaging information in treatment planning discussions. Comparative effectiveness research is demonstrating value propositions for nuclear medicine procedures relative to alternative diagnostic approaches.
Real-world evidence generation through registry programs and outcomes research supports expanded clinical adoption. Healthcare provider education initiatives are increasing awareness of appropriate radiopharmaceutical applications. Patient advocacy organizations are promoting awareness of nuclear medicine diagnostic and treatment options.
Infrastructure Development and Facility Investment
Healthcare systems are investing in nuclear medicine facility construction and modernization to meet growing clinical demand. Cyclotron installation at medical centers enables on-site radiopharmaceutical production for clinical and research applications. Radiopharmacy facility design is incorporating automation and quality systems supporting regulatory compliance. Academic medical centers are establishing centers of excellence in theranostic medicine combining clinical service, research, and education.
Equipment manufacturers are developing compact production systems suitable for hospital-based radiopharmaceutical preparation. Hot cell technology advances enable safer handling of therapeutic-level radioactive materials. Quality control instrumentation specific to radiopharmaceutical testing requirements is becoming more accessible.
Emerging Applications Beyond Oncology
Neurodegenerative disease imaging using radiopharmaceuticals targeting specific pathological proteins supports early diagnosis and treatment monitoring. Cardiac imaging applications are expanding beyond perfusion assessment to include innervation imaging and inflammation detection. Infectious disease diagnosis using pathogen-specific radiopharmaceuticals represents developing application area. Inflammatory and autoimmune conditions may benefit from molecular imaging approaches characterizing disease activity.
Research applications in drug development utilize radiopharmaceuticals to assess drug biodistribution and target engagement. Veterinary nuclear medicine is emerging as application area for companion animal diagnostics and therapy.
| Report Metric | Details |
| Report Name | and India Radiopharmaceuticals Market |
| Forecasted market size in 2029 | US$ 11660 million |
| CAGR | 8.0% |
| Forecasted years | 2023 - 2029 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Bracco Imaging, Bayer, Mallinckrodt, Nordion, Triad Isotopes, Lantheus, IBA Group, GE Healthcare, China Isotope & Radiation, Jubilant Pharma, Eli Lilly, Advanced Accelerator Applications, SIEMENS, Dongcheng, Navidea |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
Ans: The and India Radiopharmaceuticals Market size in 2029 will be US$ 11660 million.
Ans: The top 3 players hold over 40% of global market shares.
Ans: North America and Europe are main markets, they occupy over 70% of the global market. According to IMF (its July update to its World Economic Outlook), India’s GDP growth is projected at 6.1% in 2023, powered by domestic investment.
Ans: Diagnostic Radioisotopes is the main type, with a share about 90%.
Ans: Oncology is the key application, which hold over 60% shares.
Ans: The main players in the and India Radiopharmaceuticals Market are Bracco Imaging, Bayer, Mallinckrodt, Nordion, Triad Isotopes, Lantheus, IBA Group, GE Healthcare, China Isotope & Radiation, Jubilant Pharma, Eli Lilly, Advanced Accelerator Applications, SIEMENS, Dongcheng, Navidea
Ans: The Applications covered in the and India Radiopharmaceuticals Market report are Oncology, Cardiology, Other
Ans: The Types covered in the and India Radiopharmaceuticals Market report are Diagnostic Radioisotopes, Therapeutic Radioisotopes
$4350
$6525
$8700
HAVE A QUERY?
OUR CUSTOMER
