List of Tables
Table 1. Global Biopharmaceutical CDMO Service Market Size Growth Rate by Type (US$ Million): 2020 VS 2024 VS 2031
Table 2. Key Players of Cell and Gene Therapies
Table 3. Key Players of Antibodies
Table 4. Key Players of Vaccines
Table 5. Key Players of Other
Table 6. Global Biopharmaceutical CDMO Service Market Size Growth by Application (US$ Million): 2020 VS 2024 VS 2031
Table 7. Global Biopharmaceutical CDMO Service Market Size by Region (US$ Million): 2020 VS 2024 VS 2031
Table 8. Global Biopharmaceutical CDMO Service Market Size by Region (2020-2025) & (US$ Million)
Table 9. Global Biopharmaceutical CDMO Service Market Share by Region (2020-2025)
Table 10. Global Biopharmaceutical CDMO Service Forecasted Market Size by Region (2026-2031) & (US$ Million)
Table 11. Global Biopharmaceutical CDMO Service Market Share by Region (2026-2031)
Table 12. Biopharmaceutical CDMO Service Market Trends
Table 13. Biopharmaceutical CDMO Service Market Drivers
Table 14. Biopharmaceutical CDMO Service Market Challenges
Table 15. Biopharmaceutical CDMO Service Market Restraints
Table 16. Global Biopharmaceutical CDMO Service Revenue by Players (2020-2025) & (US$ Million)
Table 17. Global Biopharmaceutical CDMO Service Market Share by Players (2020-2025)
Table 18. Global Top Biopharmaceutical CDMO Service Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Biopharmaceutical CDMO Service as of 2024)
Table 19. Ranking of Global Top Biopharmaceutical CDMO Service Companies by Revenue (US$ Million) in 2024
Table 20. Global 5 Largest Players Market Share by Biopharmaceutical CDMO Service Revenue (CR5 and HHI) & (2020-2025)
Table 21. Global Key Players of Biopharmaceutical CDMO Service, Headquarters and Area Served
Table 22. Global Key Players of Biopharmaceutical CDMO Service, Product and Application
Table 23. Global Key Players of Biopharmaceutical CDMO Service, Date of Enter into This Industry
Table 24. Mergers & Acquisitions, Expansion Plans
Table 25. Global Biopharmaceutical CDMO Service Market Size by Type (2020-2025) & (US$ Million)
Table 26. Global Biopharmaceutical CDMO Service Revenue Market Share by Type (2020-2025)
Table 27. Global Biopharmaceutical CDMO Service Forecasted Market Size by Type (2026-2031) & (US$ Million)
Table 28. Global Biopharmaceutical CDMO Service Revenue Market Share by Type (2026-2031)
Table 29. Global Biopharmaceutical CDMO Service Market Size by Application (2020-2025) & (US$ Million)
Table 30. Global Biopharmaceutical CDMO Service Revenue Market Share by Application (2020-2025)
Table 31. Global Biopharmaceutical CDMO Service Forecasted Market Size by Application (2026-2031) & (US$ Million)
Table 32. Global Biopharmaceutical CDMO Service Revenue Market Share by Application (2026-2031)
Table 33. North America Biopharmaceutical CDMO Service Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 34. North America Biopharmaceutical CDMO Service Market Size by Country (2020-2025) & (US$ Million)
Table 35. North America Biopharmaceutical CDMO Service Market Size by Country (2026-2031) & (US$ Million)
Table 36. Europe Biopharmaceutical CDMO Service Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 37. Europe Biopharmaceutical CDMO Service Market Size by Country (2020-2025) & (US$ Million)
Table 38. Europe Biopharmaceutical CDMO Service Market Size by Country (2026-2031) & (US$ Million)
Table 39. Asia-Pacific Biopharmaceutical CDMO Service Market Size Growth Rate by Region (US$ Million): 2020 VS 2024 VS 2031
Table 40. Asia-Pacific Biopharmaceutical CDMO Service Market Size by Region (2020-2025) & (US$ Million)
Table 41. Asia-Pacific Biopharmaceutical CDMO Service Market Size by Region (2026-2031) & (US$ Million)
Table 42. Latin America Biopharmaceutical CDMO Service Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 43. Latin America Biopharmaceutical CDMO Service Market Size by Country (2020-2025) & (US$ Million)
Table 44. Latin America Biopharmaceutical CDMO Service Market Size by Country (2026-2031) & (US$ Million)
Table 45. Middle East & Africa Biopharmaceutical CDMO Service Market Size Growth Rate by Country (US$ Million): 2020 VS 2024 VS 2031
Table 46. Middle East & Africa Biopharmaceutical CDMO Service Market Size by Country (2020-2025) & (US$ Million)
Table 47. Middle East & Africa Biopharmaceutical CDMO Service Market Size by Country (2026-2031) & (US$ Million)
Table 48. Lonza Company Details
Table 49. Lonza Business Overview
Table 50. Lonza Biopharmaceutical CDMO Service Product
Table 51. Lonza Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 52. Lonza Recent Development
Table 53. Catalent Company Details
Table 54. Catalent Business Overview
Table 55. Catalent Biopharmaceutical CDMO Service Product
Table 56. Catalent Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 57. Catalent Recent Development
Table 58. Samsung Biologics Company Details
Table 59. Samsung Biologics Business Overview
Table 60. Samsung Biologics Biopharmaceutical CDMO Service Product
Table 61. Samsung Biologics Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 62. Samsung Biologics Recent Development
Table 63. FUJIFILM Diosynth Biotechnologies Company Details
Table 64. FUJIFILM Diosynth Biotechnologies Business Overview
Table 65. FUJIFILM Diosynth Biotechnologies Biopharmaceutical CDMO Service Product
Table 66. FUJIFILM Diosynth Biotechnologies Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 67. FUJIFILM Diosynth Biotechnologies Recent Development
Table 68. Boehringer Ingelheim Company Details
Table 69. Boehringer Ingelheim Business Overview
Table 70. Boehringer Ingelheim Biopharmaceutical CDMO Service Product
Table 71. Boehringer Ingelheim Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 72. Boehringer Ingelheim Recent Development
Table 73. WuXi Biologics Company Details
Table 74. WuXi Biologics Business Overview
Table 75. WuXi Biologics Biopharmaceutical CDMO Service Product
Table 76. WuXi Biologics Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 77. WuXi Biologics Recent Development
Table 78. Recipharm Company Details
Table 79. Recipharm Business Overview
Table 80. Recipharm Biopharmaceutical CDMO Service Product
Table 81. Recipharm Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 82. Recipharm Recent Development
Table 83. Thermo Fisher Scientific Company Details
Table 84. Thermo Fisher Scientific Business Overview
Table 85. Thermo Fisher Scientific Biopharmaceutical CDMO Service Product
Table 86. Thermo Fisher Scientific Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 87. Thermo Fisher Scientific Recent Development
Table 88. AGC Biologics Company Details
Table 89. AGC Biologics Business Overview
Table 90. AGC Biologics Biopharmaceutical CDMO Service Product
Table 91. AGC Biologics Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 92. AGC Biologics Recent Development
Table 93. Rentschler Biopharma Company Details
Table 94. Rentschler Biopharma Business Overview
Table 95. Rentschler Biopharma Biopharmaceutical CDMO Service Product
Table 96. Rentschler Biopharma Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 97. Rentschler Biopharma Recent Development
Table 98. KBI Biopharma Company Details
Table 99. KBI Biopharma Business Overview
Table 100. KBI Biopharma Biopharmaceutical CDMO Service Product
Table 101. KBI Biopharma Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 102. KBI Biopharma Recent Development
Table 103. Siegfried Company Details
Table 104. Siegfried Business Overview
Table 105. Siegfried Biopharmaceutical CDMO Service Product
Table 106. Siegfried Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 107. Siegfried Recent Development
Table 108. Aenova Group Company Details
Table 109. Aenova Group Business Overview
Table 110. Aenova Group Biopharmaceutical CDMO Service Product
Table 111. Aenova Group Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 112. Aenova Group Recent Development
Table 113. GenScript Company Details
Table 114. GenScript Business Overview
Table 115. GenScript Biopharmaceutical CDMO Service Product
Table 116. GenScript Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 117. GenScript Recent Development
Table 118. ProBioGen Company Details
Table 119. ProBioGen Business Overview
Table 120. ProBioGen Biopharmaceutical CDMO Service Product
Table 121. ProBioGen Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 122. ProBioGen Recent Development
Table 123. Northway Biotech Company Details
Table 124. Northway Biotech Business Overview
Table 125. Northway Biotech Biopharmaceutical CDMO Service Product
Table 126. Northway Biotech Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 127. Northway Biotech Recent Development
Table 128. 3P Biopharmaceuticals Company Details
Table 129. 3P Biopharmaceuticals Business Overview
Table 130. 3P Biopharmaceuticals Biopharmaceutical CDMO Service Product
Table 131. 3P Biopharmaceuticals Revenue in Biopharmaceutical CDMO Service Business (2020-2025) & (US$ Million)
Table 132. 3P Biopharmaceuticals Recent Development
Table 133. Research Programs/Design for This Report
Table 134. Key Data Information from Secondary Sources
Table 135. Key Data Information from Primary Sources
Table 136. Authors List of This Report
List of Figures
Figure 1. Biopharmaceutical CDMO Service Picture
Figure 2. Global Biopharmaceutical CDMO Service Market Size Comparison by Type (2020-2031) & (US$ Million)
Figure 3. Global Biopharmaceutical CDMO Service Market Share by Type: 2024 VS 2031
Figure 4. Cell and Gene Therapies Features
Figure 5. Antibodies Features
Figure 6. Vaccines Features
Figure 7. Other Features
Figure 8. Global Biopharmaceutical CDMO Service Market Size by Application (2020-2031) & (US$ Million)
Figure 9. Global Biopharmaceutical CDMO Service Market Share by Application: 2024 VS 2031
Figure 10. SMBs Case Studies
Figure 11. Large Companies Case Studies
Figure 12. Biopharmaceutical CDMO Service Report Years Considered
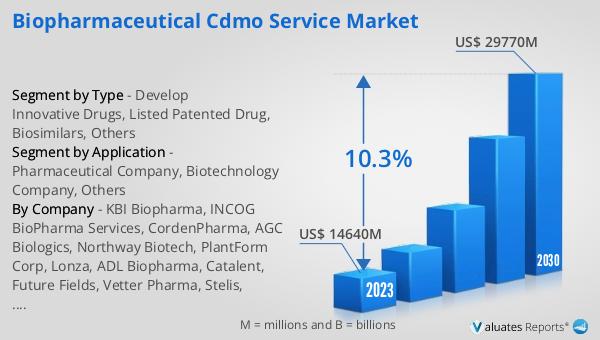
Figure 13. Global Biopharmaceutical CDMO Service Market Size (US$ Million), Year-over-Year: 2020-2031
Figure 14. Global Biopharmaceutical CDMO Service Market Size, (US$ Million), 2020 VS 2024 VS 2031
Figure 15. Global Biopharmaceutical CDMO Service Market Share by Region: 2024 VS 2031
Figure 16. Global Biopharmaceutical CDMO Service Market Share by Players in 2024
Figure 17. Global Top Biopharmaceutical CDMO Service Players by Company Type (Tier 1, Tier 2, and Tier 3) & (based on the Revenue in Biopharmaceutical CDMO Service as of 2024)
Figure 18. The Top 10 and 5 Players Market Share by Biopharmaceutical CDMO Service Revenue in 2024
Figure 19. North America Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 20. North America Biopharmaceutical CDMO Service Market Share by Country (2020-2031)
Figure 21. United States Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 22. Canada Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 23. Europe Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 24. Europe Biopharmaceutical CDMO Service Market Share by Country (2020-2031)
Figure 25. Germany Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 26. France Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 27. U.K. Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 28. Italy Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 29. Russia Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 30. Nordic Countries Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 31. Asia-Pacific Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 32. Asia-Pacific Biopharmaceutical CDMO Service Market Share by Region (2020-2031)
Figure 33. China Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 34. Japan Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 35. South Korea Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 36. Southeast Asia Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 37. India Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 38. Australia Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 39. Latin America Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 40. Latin America Biopharmaceutical CDMO Service Market Share by Country (2020-2031)
Figure 41. Mexico Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 42. Brazil Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 43. Middle East & Africa Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 44. Middle East & Africa Biopharmaceutical CDMO Service Market Share by Country (2020-2031)
Figure 45. Turkey Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 46. Saudi Arabia Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 47. UAE Biopharmaceutical CDMO Service Market Size YoY Growth (2020-2031) & (US$ Million)
Figure 48. Lonza Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 49. Catalent Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 50. Samsung Biologics Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 51. FUJIFILM Diosynth Biotechnologies Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 52. Boehringer Ingelheim Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 53. WuXi Biologics Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 54. Recipharm Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 55. Thermo Fisher Scientific Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 56. AGC Biologics Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 57. Rentschler Biopharma Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 58. KBI Biopharma Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 59. Siegfried Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 60. Aenova Group Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 61. GenScript Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 62. ProBioGen Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 63. Northway Biotech Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 64. 3P Biopharmaceuticals Revenue Growth Rate in Biopharmaceutical CDMO Service Business (2020-2025)
Figure 65. Bottom-up and Top-down Approaches for This Report
Figure 66. Data Triangulation
Figure 67. Key Executives Interviewed